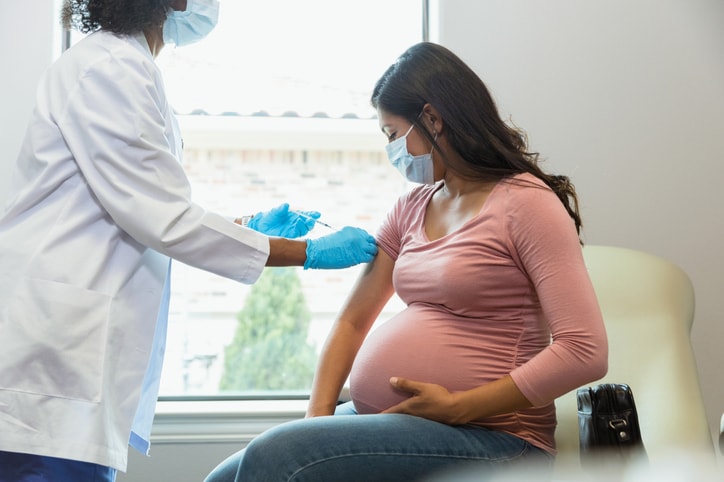
Earlier this week, the U.S. Food and Drug Administration (FDA) approved Abrysvo, Pfizer’s respiratory syncytial virus (RSV) vaccine, for use in pregnant individuals. The drug was first approved in May for adults 60 and older, but now has been authorized for pregnant people during their third trimester after trials showed antibodies were effectively passed onto babies through the placenta.
The main objective of the vaccine: “Protection of babies after they’re born,” says Dr. Christy Evans, a board-certified OB/GYN at Almond OB/GYN in Los Angeles, who adds, “Most of us have been infected by the time we’re around 2 years old, usually with a mild case, so we end up having some level of immunity. But babies are particularly susceptible to more severe disease, which is why having an in utero option is so beneficial.”
How dangerous is RSV in newborns?
RSV is the number one cause of hospitalization in newborns in the U.S., with approximately 58,000 to 80,000 children under 5 – mostly babies under a year — being hospitalized for it each year. For perspective, about 20,000 children under 5 are hospitalized in the U.S. each year for flu.
RSV, which is a common respiratory virus, typically goes away on it’s own after a week or two, according to the Centers for Disease Control (CDC), but for infants, especially those 6 months and under, it can be particularly dangerous, as their airways are so small, making it difficult to breath when there’s any amount of swelling. It’s also worth noting that one recently-published study of infants who were admitted to the ICU with RSV in late 2022 found that the majority were born full-term and previously healthy.
This past, post-pandemic winter proved to be a particularly robust RSV season, and was a contributing factor to the “tripledemic” (RSV, flu and COVID) that overwhelmed the health care system.
How does the RSV vaccine for pregnant persons protect babies?
Similar to a flu shot, Abrysvo passes antibodies to babies through the placenta, boosting their immunity to the virus.
In a clinical trial of almost 7,400 participants, Abrysvo lowered the risk of severe disease from RSV in infants by 82% within about three months after birth. The efficacy dropped to about 69% by six months.
In July, the FDA also approved Beyfortus (nirsevimab-alip), which is a monoclonal antibody therapy from Astra-Zeneca for infants up to 8 months of age (antibody treatments produce prefabricated antibodies, whereas vaccines teach the body how to make them).
While there’s no cut-and-dried answer as to which is better — Abrysvo or Beyfortus — Evans suspects the former may be more effective.
“There haven’t been studies evaluating the two options’ relative benefits, but the maternal vaccination may produce a broader antibody response, as the vaccination works in a similar way to the body’s natural immune response against a virus,” she explains. “This means that it may be more effective at targeting RSV than an antibody shot. But we don’t yet have data to support that theory.”
— Dr. Christy Evans, a board-certified OB/GYN at Almond OB/GYN in Los Angeles
“Currently, the Pfizer vaccination [Abrysvo] is approved for pregnant women at 32-36 weeks gestation. The vaccination is not yet available to mothers in the U.S. for this purpose. We are awaiting a final CDC recommendation on who will be able to receive it. I expect we’ll be hearing about that sometime in October.”
Can any pregnant person get the vaccine?
No, any pregnant person cannot get the RSV vaccine. “Currently, the Pfizer vaccination [Abrysvo] is approved for pregnant women at 32-36 weeks gestation,” Evans says, adding: “The vaccination is not yet available to mothers in the U.S. for this purpose. We are awaiting a final CDC recommendation on who will be able to receive it. I expect we’ll be hearing about that sometime in October.”
Put another way: While the FDA has technically approved the drug for the third trimester, it still has to go through an advisory committee and the CDC has to recommend who should receive it.
What are the side effects of Abrysvo?
In the clinical trial, the most common side effects reported by pregnant women were:
- Fatigue.
- Headache.
- Some injection site pain.
- Muscle pain.
- Joint pain.
- Nausea.
Severe side effects, such as pre-eclampsia in pregnant people, low birth weight and jaundice in infants and preterm birth (5.7% compared to 4.7%), were slightly higher in the vaccine group compared to the placebo.
The FDA noted that they were unable to “establish or exclude a causal relationship between preterm birth and Abrysvo;” however, they advise the vaccine be given 32-36 weeks to mitigate possible risks.
The bottom line on the RSV vaccine
While Abrysvo isn’t on the shelves for pregnant people yet, it should be within the next few months; however, in the early stages, some may be given priority to vaccine over others. The good news is that there are now two FDA-approved treatments against severe RSV infants before peak season.